Orthocell reports high success rate from CelGro surgical trial on patients with rotator cuff injuries

Patients were able to regain normal shoulder function following CelGro tendon regeneration surgery.
Follow-up results from a CelGro tendon regeneration clinical trial conducted by Perth-based Orthocell (ASX: OCC) has confirmed all patients achieved a successful tendon repair with no need for revision surgeries.
Conducted by St John of God Hospital Subiaco and the University of Western Australia, the trial involved patients who had previously suffered full-thickness tears of the rotator cuff tendon in the shoulder following work-related, motor vehicle or sporting injuries.
Patients experienced chronic pain and difficulty performing daily activities such as sleeping, bathing and dressing, as well as playing sport or working.
Interim results
Interim results in June 2018 involving the first 10 patients found the CelGro naturally-derived collagen membrane to be safe, tolerable and capable of guiding healing in the surgical repair of the rotator cuff tendon.
A clinical follow-up two years after surgery found those patients achieved a successful tendon repair, reporting a return to full range of movement and improved quality of life with no pain.
There were no adverse events related to CelGro and no evidence of systemic adverse reaction or side-effects at the implant site.
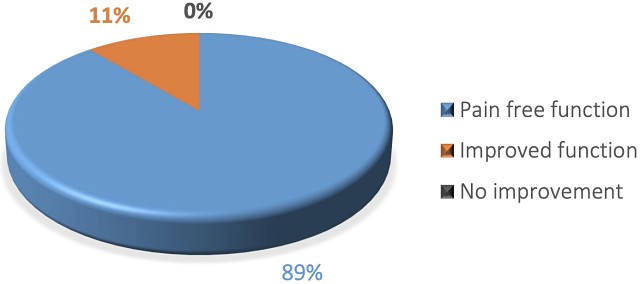
CelGro tendon regeneration trial results.
Importantly, no patients required further surgery for a re-tear of the rotator cuff tendon – a critical finding since revision surgeries for re-tears are believed to occur in up to 57% of cases.
The clinical study was designed to assess the performance of CelGro tendon regeneration technology when used to augment surgical repair of rotator cuff tendinopathy and tear.
Pain measurement
Results of the 10 patients in the trial showed CelGro can guide and strengthen tendon healing when used in the surgical repair of the rotator cuff tendon in the shoulder, with 89% of patients returning to pain-free function.
Improvements in function and reductions in pain following treatment were measured using the American Shoulder and Elbow Society (ASES) scorecard from zero to 100, with a higher score indicating a better outcome.
Before surgery with CelGro, patients recorded an average ASES score of 65, with significant chronic pain and difficulty performing daily activities.
One year post-surgery, the average score had increased to a more positive 94, with patients reporting significant improvements in daily activities such as brushing their hair with the affected arm, getting dressed and sleeping at night.
Many patients had returned to work and sport without discomfort or pain.
Commercialisation strategy
Orthocell managing director Paul Anderson said the company has a clear commercialisation strategy for CelGro and intends to leverage the trial results and the technology’s recent European approval for regulatory submission in key regions.
“CelGro is proving to be a breakthrough soft tissue reconstruction platform technology with positive data announced now in nerve, tendon and bone applications,” he said.
“We are extremely excited by the clinical follow-up results which indicate effectiveness of CelGro in reducing the surgical revision rate.”
https://www.youtube.com/watch?v=BG_TMyHur74
In late 2018, Orthocell completed a pre-submission meeting with the US Food and Drug Administration as a first step towards gaining approval to market CelGro in the US.
Chronic condition
Rotator cuff tears can be a source of chronic shoulder pain, reduced strength and general weakness – in the US alone, more than 500,000 repair surgeries are performed each year.
Data presented in 2015 by the American Academy of Orthopaedic Surgeons found that, across a group of 500 patients, re-tears and revision surgeries can occur in up to 57% of cases.
“Shoulder pain affects more than 50% of adults over the age of 50 [and] most frequently, is the result of damage to the rotator cuff tendon,” Mr Anderson said.
“Surgery to repair rotator cuff tendons is problematic because the underlying tendon is weakened and re-tears requiring additional surgery are very common.”
He said Orthocell designed CelGro with handling characteristics to assist surgeons perform reconstructive procedures while creating a unique healing environment to strengthen each repair.
“This trial provides confidence that the use of CelGro may reduce the estimated re-tear rate that occurs with the commonly-used surgical approach,” Mr Anderson said.
Global market
CelGro’s global addressable market in rotator cuff tendon repairs is estimated to be worth approximately US$1 billion per year.
Market growth is expected to be underpinned by the surgical industry’s preference for high-quality membranes which achieve more effective and faster results for patients.
The technology is also being used in clinical studies to assist in rejoining severed, or damaged peripheral nerves and the repair of cartilage defects.
EU approval
In 2017, Orthocell received European Union authorisation for the use of CelGro collagen scaffold medical device for dental bone and soft tissue applications.
Mr Anderson said that authorisation validated CelGro’s “quality manufacturing [and] product performance” and provided a strong foundation for further regulatory approvals.
“European regulatory approval marks a major milestone for [us] as it enables commercial roll-out in the lucrative dental bone and soft tissue regeneration market, where there is a significant and growing demand and market opportunity,” he said at the time.
CelGro’s dental addressable market is estimated to be worth more than US$600 million per year across approximately 1.5 million procedures.
Market growth is expected to be underpinned by an ageing population, growing demand for dental therapies and the industry preference for functional bio-absorbable membranes.
At midday, shares in Orthocell were trading 11.39% higher at $0.44.
