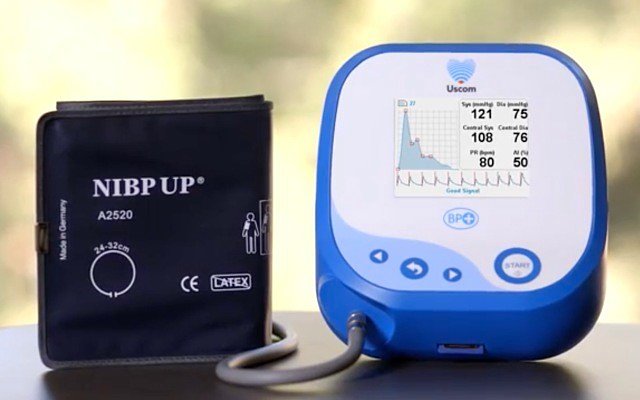
Australian medical technology company Uscom (ASX: UCM) has been granted four new copyrights and four trademarks by the Chinese government in relation to its 1A, BP+ and SpiroSonic medical devices.
The copyrights were issued by the National Copyright Administration, while the trademarks were published by the Trademark Office for the State Administration of Industry & Commerce.
They cover seven new BP+ blood pressure monitors and SpiroSonic digital ultrasonic spirometers which are currently in the final stages of regulatory review with China’s National Medical Products Authority.
Uscom’s 1A cardiovascular monitor is already approved for sale in China and is responsible for more than 60% of Uscom’s total annual revenue.
Ongoing protection
Generated out of Uscom’s newly-registered office in Beijing, the copyright and trademark applications will provide ongoing commercial protection of the company’s products as new devices and markets are approved.
Supporting patents are also in review and will provide a secondary intellectual property strategy for protecting opportunities arising from market development activities in China.
Uscom associate professor Rob Phillips said the applications were granted following a decade’s worth of work addressing China’s health market, estimated to comprise 1.4 billion people, 4.8 million doctors and registered nurses and 30,000 hospitals.
“Intellectual property is our asset, and protection of our anticipated revenue in the market place is vital particularly in China, [which is] the largest and fastest growing medical device market in the world,” he said.
Health monitors
The 1A device has been marketed as an easy-to-use, advanced haemodynamic monitor which measures cardiovascular function and detects irregularities, with lifesaving applications in paediatrics, emergency and intensive care, and anaesthesia.
The supra-systolic, oscillometric BP+ monitor is one of the only devices available to measure blood pressure and pulse pressure waveforms at the heart and arm, collecting information previously only available using invasive cardiac catheterisation.
Uscom claims the device to be “the emerging standard of care measurement” in hypertension, heart failure and vascular health, and is supported by BP+ reporter software which provides a digital platform to archive patient examinations, map progress over time, analyse pulse pressure waves and generate summary reports.
The company is also setting standards of pulmonary assessment with its high-fidelity SpiroSonic devices and digital interfacing SpiroReporter which can be employed in a variety of settings to define and guide improved management in asthma, chronic obstructive pulmonary disease and occupational lung disease.
Market growth
Industry research suggests the global spirometry market is growing at around 10% per annum and is projected to exceed US$1 billion per annum by 2022.
The growth is largely related to global increases in the incidence of asthma, COPD and OLD, both associated with significant mortality and morbidity.
Mr Phillips said Uscom’s partnership with experienced regulatory and intellectual property management operators in China will help the company with its expansion into the country’s growing healthcare market.
“This pathway and the infrastructure we are building in China will become a conduit for new products for years to come,” he said.
At midday, shares in Uscom were trading 3.70% higher at $0.140.