ANP.ASX
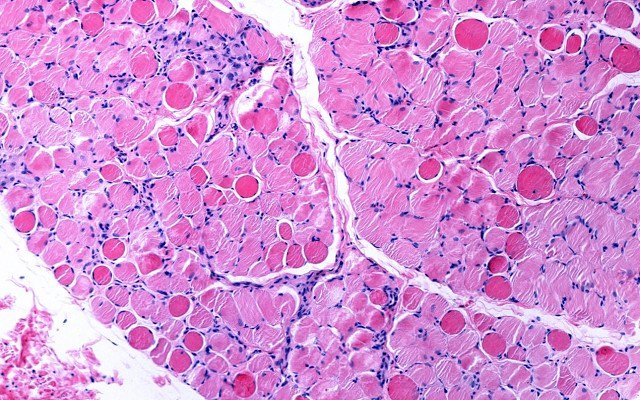
Antisense Therapeutics reports ‘strong initial efficacy’ for Duchenne Muscular Dystrophy drug in Phase 2 trial
Biopharmaceutical company Antisense Therapeutics (ASX: ANP) emerged from a trading halt to announce that its immunomodulatory therapy, ATL1102 for Duchenne Muscular Dystrophy (DMD) had met the required safety standards and achieved “strong initial efficacy” as part of its Phase 2 clinical trial. Trial results were also vocally supported by a slue of scientists, researchers and other […]

Antisense Therapeutics set to widen its scope of treatment options
Following recently reported positive clinical trial results relating to its research into Duchenne Muscular Dystrophy, biopharmaceutical drug development company Antisense Therapeutics (ASX: ANP) has declared that it’s now actively exploring clinical development opportunities in other indications where inflammation plays a key role in disease progression. The company’s overarching mission is to develop and commercialise novel […]

Clinical trial confirms Antisense Therapeutics drug can inhibit progression of Duchenne muscular dystrophy
Data from a phase 2 clinical trial by Antisense Therapeutics (ASX: ANP) on the immunomodulatory therapy ATL1102 for Duchenne muscular dystrophy has confirmed the drug can have positive effects on progression of the disease. The trial aimed to assess the safety and tolerability of 25 milligram doses of ATL1102 administered to nine wheelchair-bound young male […]

Antisense reports positive early results from clinical trial of new drug to treat patients with Duchenne muscular dystrophy
A preliminary review of a phase two clinical trial involving an immunomodulatory therapy drug developed by Antisense Therapeutics (ASX: ANP) has shown a positive effect for patients with non-amubulant Duchenne muscular dystrophy. The review of six patients who had completed 24 weeks of treatment with the drug ATL1102 indicated a positive effect at an immunomodulatory […]