RADIOPHARM THERANOSTICS ORD

Radiopharm Theranostics Doses First Patient in RV-01 Phase 1/2a Trial for Aggressive Cancers
RAD doses first patient in RV-01 Phase 1/2a trial for aggressive cancers, a 177Lu-radiotherapeutic targeting B7-H3, to define dose for future studies.

Radiopharm Theranostics Granted Ethics Approval for RAD402 Phase 1 Trial to Treat Advanced Prostate Cancer
Radiopharm Theranostics (ASX: RAD) has received Bellberry human research ethics committee approval in Australia for a Phase 1 first in-human clinical trial of RAD402 for the treatment of metastatic or locally-advanced prostate cancer.
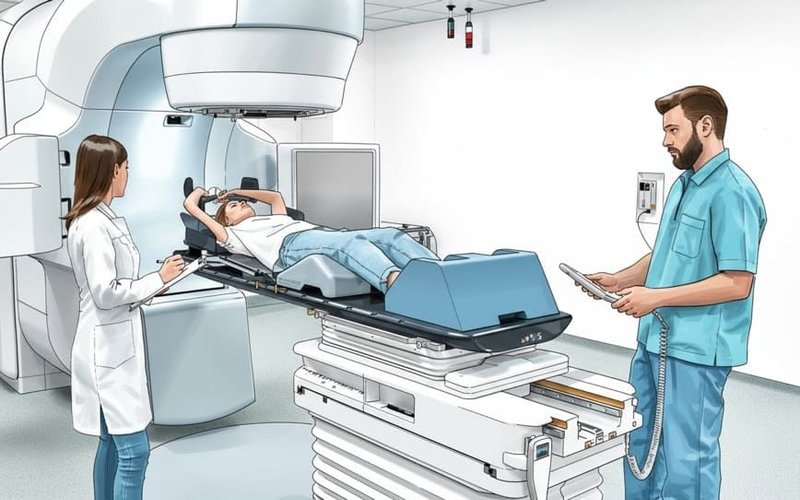
Starpharma and Radiopharm Theranostics Partner to Develop Novel Radiotherapy Asset
Starpharma Holdings (ASX: SPL) has entered into a research and option partnership with Radiopharm Theranostics (ASX: RAD) to develop and manufacture a dendrimer drug conjugate incorporating a radiopharmaceutical molecule to target certain cancers. The partnership, which follows six months of preliminary research under Starpharma’s Star Navigator drug development program, will see Radiopharm pay Starpharma a $500,000 for an […]

Radiopharm Theranostics Receives FDA Investigational New Drug Clearance for RV-01 Cancer Therapy
Radiopharm Theranostics (ASX: RAD) has received investigational new drug clearance from the US Food and Drug Administration (FDA) for therapeutic candidate Betabart (RV-01). RV-01 targets the B7H3 immune checkpoint molecule that plays a role in cancer development and progression. The FDA clearance will now allow the company to initiate a first-in-human Phase 1 clinical trial […]