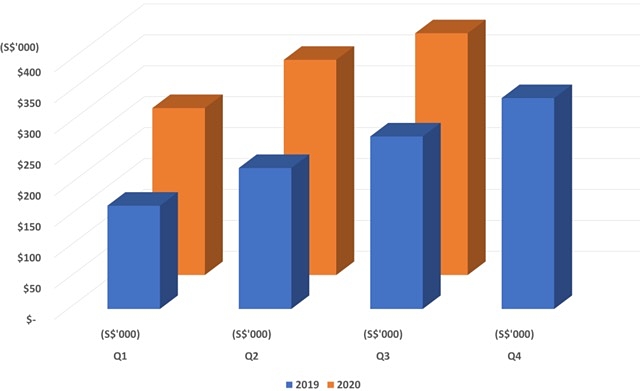
Medical technology company Osteopore (ASX: OSX) revealed it experienced “encouraging sales growth” with quarterly revenue growing to over $408,000, despite challenges posed by COVID-19.
The feat represents a 40% increase over the previous corresponding period and, according to Osteopore, highlights the consistent growth in demand for the company’s unique 3D printed bioresorbable implants.
Strong revenue figures have also pushed the company’s total sales for the year to over S$1 million (A$1.04 million) while matching 2019’s total revenue haul in just the first three quarters of 2020.
Currently, Osteopore is commercialising a range of bespoke products specifically engineered to facilitate bone healing.
Osteopore’s patented technology can fabricate specific micro-structured scaffolds for bone regeneration through 3D printing and bioresorbable material.
The key differentiator is that its patent-protected scaffolds are made from proprietary polymer formulations that naturally dissolve over time, thereby creating better outcomes for patients including healthy bone tissue and significantly reducing post-surgery complications that are commonly associated with permanent implants.
Continued rising sales
One of the key reasons behind Osteopore’s strong sales boost over the past six months has been its focus on Europe.
The September period saw the company raise its sales to the EU including Italy, Greece and the Netherlands as it rolled-out its products into these new markets.
Meanwhile, in Singapore, Osteopore has benefited from being designated as an “essential service” in the country, thereby allowing it to remain operational throughout the pandemic while executing its growth strategy.
The company also completed its first shipments to Italy, Indonesia and Taiwan.
Further positive performance news came from the US during the period.
Osteopore reported that the agreement it secured in July with American cranial bone fixation specialist Bioplate is actively promoting the sale of its products in the US market.
Bioplate has begun marketing Osteopore’s range of bespoke products across California, Texas, Wyoming, Ohio, Arizona, Indiana and Puerto Rico.
To further clarify its stateside strategy, Osteopore explained that it aims to sign additional commercial partnerships to distribute products into other US regions not covered by Bioplate.
Osteopore expects revenue growth to continue as it increases its presence in multiple geographic territories and expands manufacturing capability to meet future demand.
Capital raise
To continue its incremental expansion ambitions and establish a presence in more territories, Osteopore went to the market in August to raise $8.5 million.
The placement was supported by a number of the company’s existing shareholders and introduced a number of new, high net-worth investors and institutions to its register.
The newly raised capital is expected to be deployed to consolidate Osteopore’s existing revenue base and supporting ongoing clinical trials for dental and orthopaedic applications, the company said.
Moreover, the funds will assist Osteopore with development of “second-generation products and complementary technologies that support potential future business growth”, the company said.
Experimental development
Potentially a key development in terms of long-term outlooks, Osteopore penned an exclusive option in July to licence novel 3D printed modular bone implant technology being developed at the Queensland University of Technology (QUT).
According to Osteopore, the technology complements its existing bone regenerating products and has shown early-stage success in regrowth of long bone defects in patients who have lost more than 6cm of bone to injury or disease.
