Osteopore (ASX: OSX) is now be able to make its 3D-printed medical scaffolds widely available across Australia after its three craniofacial products were approved by the Therapeutic Goods Administration (TGA).
The bone healing biotech company emerged from a trading halt this morning to announce its Osteomesh, Osteoplug and Osteoplug-C products have now been registered on the Australian Register of Therapeutic Goods. The products are also expected to be included in the Prosthesis Listing in July 2020.
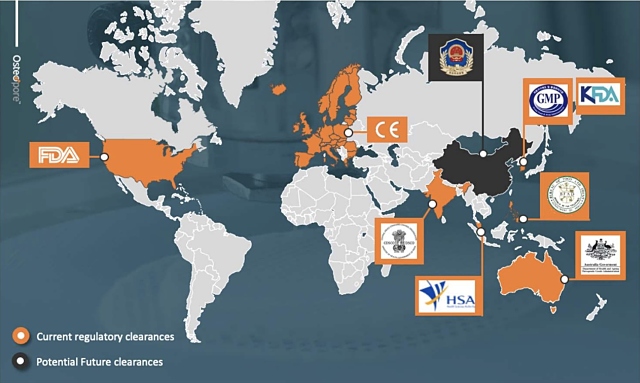
This latest approval adds to the company’s list of regulatory clearances in international markets including Food and Drug Administration (FDA) approval in the United States, CE Mark in Europe, as well as Singapore and other East Asian approvals.
Expansion in Australia
Osteopore’s products have been successfully used as customised implants in Australia before, but only under the Special Access Scheme.
“Obtaining TGA regulatory approval now allows Osteopore to make its products more broadly commercially available to doctors and hospitals across Australia, and the company will continue ongoing discussions with potential distribution partners,” it stated.
Osteopore chief executive officer Goh Khoon Seng said the approval has now opened up the Australian market to the company.
“Australia is a key market for the global expansion of our business, and we are excited to be able to continue to build our revenue streams in this market,” he said.
The company has already appointed a locally-based business development consultant to lead its distribution strategy and drive sales in Australia.
In addition to Australia, Osteopore’s revenue expansion strategy is aimed at growing revenue from existing Asian markets and establishing new markets in United States, China and Europe.
Bone healing technology
Osteopore’s unique, patented 3D-printed scaffolds are designed to naturally dissolve over time to leave only natural, healthy bone tissue.
The company claims its technology significantly reduces the risk of post-surgery complications that are commonly associated with permanent bone implants.
The company’s craniofacial products are used as fillers over bone voids and have a broad application in neurological and craniofacial surgery.
Specifically, Osteomesh can treat orbital floor fractures and Osteoplug is used to regenerate bone tissue over neurosurgical burr holes and other cranial defects.
Osteoplug-C can be used in similar applications as Osteoplug but in conjunction with cerebral shunt operations.
Manufacturing continues amid COVID-19 restrictions
Earlier this month, Osteopore reassured investors its sales had not been significantly impacted by COVID-19 containment measures.
Osteopore executive director Geoff Pocock told Small Caps the company’s materials supply chain has not been affected as its Singapore-based manufacturing facility is deemed an ‘essential’ operation relating to healthcare.
“We have much more control over our supply chain and our manufacturing process… we’re not reliant on international supply chains to the same degree as a lot of other companies, who are finding supply chains a real challenge,” he said.
Osteopore also said in its 8 April announcement that future sales growth would be supported by expanding production capacity.
