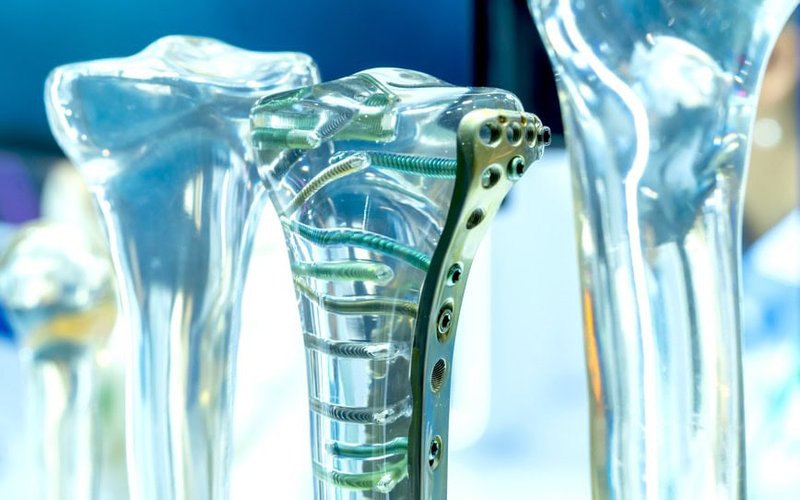
Regenerative medicine company Osteopore (ASX: OSX) has secured European Union medical device regulation approval for its custom orthopaedic and cranial implants.
The approval unlocks opportunities for the company to offer high-value custom implants to the European market to complement its range of off-the-shelf neurosurgical and craniofacial implants.
Osteopore will distribute the products via an exclusive agreement with US orthopaedic solutions company Zimmer Biomet and expected the deal to generate new revenue streams for the company.
Improving access
Osteopore chief executive officer Dr Yujing Lim said the European approval was part of the company’s strategy towards improving access to its high-value solutions.
“Custom cranial implant approval creates an opportunity to deepen our relationship with Zimmer Biomet, which now has access to our off-the-shelf and custom implant offerings,” he said.
“Our custom implants have already demonstrated transformational effects on patients in earlier publications and, with this approval, we can now boost the reach of our orthopaedic and cranial implants in Europe.”
European market size
Analysts currently value the European custom cranial implant market at $618.4 million and project a 9.4% compound annual growth rate out to 2030.
The increased prevalence of cranial surgeries due to road accidents, sports injuries and conditions such as brain tumours are seen as the primarily drivers of this growth.
The European custom orthopaedic implant market is expected to reach $26.7 billion by 2029, driven by an ageing population and rising demand for advanced devices which enhance mobility and decrease surgical recovery times.
Orthopaedic agreement
Osteopore recently signed a five-year sales and marketing agreement with Swiss holding company DiethelmKellerSiberHegner (DKSH) to promote its off-the-shelf orthopaedic products in Singapore.
The centrepiece of the Singapore expansion is Osteopore’s high tibial osteotomy (HTO) product to support knee preservation surgeries at Singapore General Hospital.
These doubled from 50 cases in 2020 to approximately 100 in 2021.
Degenerative condition
Degenerative joint condition knee osteoarthritis impacts more than 10% of adults in the region aged between 40 and 60.
Knee preservation has the highest rate of success in relieving symptoms and delaying more invasive surgeries such as total knee replacement.
“Partnering with a company which boasts the channel access, resources and experience of DKSH is a significant opportunity to commercialise our products in a meaningful and sustainable way,” Dr Lim said.
“Early clinical results for HTO provide us with the confidence to launch a quality product and we look forward to growing this market segment collaboratively with DKSH.”