Biotech company Noxopharm (ASX: NOX) will seek approval from the US Food and Drug Administration for a clinical study involving its end-stage prostate cancer drug Veyonda on early-stage COVID-19 patients with the aim of blocking the progression of the disease.
Noxopharm believes the drug’s active ingredient idroxonil could potentially block the hyper-inflammation which is believed to be responsible for deaths in patients with COVID-19 infection.
Idronoxil is a small molecule with multiple anti-cancer mechanisms.
Inhibition of the sphingosine-1-phosphate (S1P) and STING (stimulator of interferon genes) signalling pathways are thought to be the two principal factors, both of which play key roles in regulating the immunological and inflammatory responses in tumours.
Overwhelming condition
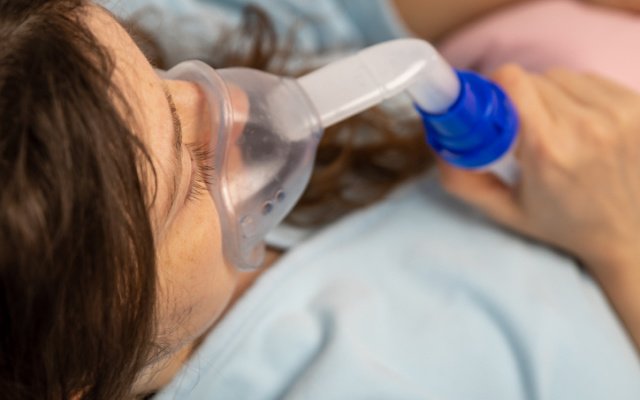
The danger with COVID-19 infection lies in its progression from a mild disease into an overwhelming condition characterised by respiratory and multi-organ failure, clotting problems and septic shock.
To date there is no approved treatment for the disease and the patient death rate continues to be high.
Medical intervention at the end-stage of COVID-19 limited to supportive treatment including antibiotics and the use of ventilators.
Pathway blocking
Noxopharm plans to use Veyonda to block STING signalling, which in some COVID-19 patients is thought to contribute to the self-destruction of major organs.
The finding was discovered in studies at Victoria’s Hudson Institute of Medical Research which showed that idronoxil can block the STING signalling pathway associated with cellular damage of the kind caused by poor oxygen levels (or hypoxia).
Noxopharm chief executive officer Graham Kelly said the timing of Hudson’s research and the planned clinical trial was right.
“With the emerging possibility that an abnormally high STING response is a factor in COVID-19 death, having an inhibitor of STING signalling ready to be tested in [these] patients is a considerable responsibility,” he said.
“Proving the value of Veyonda to COVID-19 patients is a humanitarian and regulatory approval opportunity that we cannot overlook.”
The STING effect
STING is part of a primitive defence mechanism in humans which detects the presence of invading pathogenic organisms such as viruses or bacteria and plays an important role in the clearance of damaged cells and tissues.
Both responses involve the production of proteins known as cytokines which co-ordinate subsequent immune and tissue repair (inflammatory) responses.
STING engagement in the early stages of infections can contribute positively to the body’s immune response to some pathogens.
It can become a negative and self-destructive force if the infection persists and progresses to the point of causing extensive tissue damage.
Under those conditions, the STING pathway contributes to a ‘cytokine storm’ along with the production of blood clotting factors, all of which promote further organ damage and septic shock.
According to Hudson research, a cytokine storm trigger for COVID-19 patients can be increased tissue damage associated with hypoxia stemming from poor lung function.
High levels of cytokines and clotting factors are proving to be a predictor of their mortality.
“The need to prevent the phenomena of cytokine storm and [resulting] septic shock in COVID-19 patients looks likely to remain for some considerable time, and may even remain a long-term need should development of an effective vaccine prove challenging,” Mr Kelly said.
Oncology drug
Mr Kelly said Veyonda is first and foremost an oncology drug, with end-stage prostate cancer remaining its primary focus.
“Any clinical studies in non-oncology patients will require non-dilutive funding, which [we] believe in the current environment should be achievable once we receive the go-ahead [for the trial],” he said.
“Veyonda has the added advantage of having addressed the issue of safety by proving to be well-tolerated in patients with advanced cancers and poor quality of life.”
Noxopharm is currently obtaining guidance from the USFDA on the appropriate regulatory approval pathway to pursue in relation to COVID-19 patients.
The company also is pursuing the option of testing Veyonda in patients suffering septic shock from a range of infective agents.