MGC Pharma breaks into Australian pharmaceutical market with epilepsy drug

MGC Pharmaceuticals (ASX: MXC) has inked a heads of agreement with Australian distributor HL Pharma Pty Ltd to distribute its CannEpil product to Australian epilepsy patients from early 2018.
This is the latest commercial agreement the company has bagged after announcing on Monday it had executed the first part of its A$40 million agreement with South Korean cosmetics manufacturer Varm Cosmo, securing MGC Pharmaceuticals an initial A$8 million for supplying its cannabidiol products.
Under the terms of today’s agreement with HL Pharma, the companies will work collaboratively to import and distribute CannEpil to Australian hospitals and pharmacies.
HL Pharma will gain the necessary approvals from the Therapeutic Goods Agency and Australia’s Office of Drug Control for importation and supply of the product, as well as marketing.
MGC Pharmaceuticals is currently manufacturing its first CannEpil batch to meet HL Pharma’s first order of 170 bottles. MGC Pharmaceuticals anticipates shipping the maiden batch within four months.
The company’s co-founder and chief executive officer Roby Zomer said the agreement with HL Pharma set the timeline for bringing MGC Pharmaceuticals’ first medical cannabis products to Australia.
Mr Zomer added CannEpil will be an “affordable” treatment option for Australian epilepsy sufferers and sold as a 50ml bottle containing cannabidiol and cannabinoids.
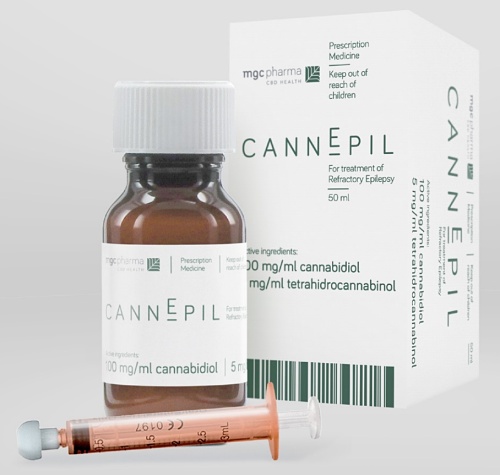
MGC Pharma’s CannEpil epilepsy treatment product.
Administered orally, it is planned the CannEpil will retail at less than A$800, which the company claims is ‘significantly lower’ that current competing product.
As part of the agreement, MGC Pharmaceuticals will leverage its existing relationships with Epilepsy Action Australia to fast-track an Australian patient market through building multiple prescribing doctors.
With less than 100 patients currently registered, the deal is anticipated to bring in A$1 million in initial revenue.
Epilepsy Action Australia claims about 25,000 Australians are diagnosed with epilepsy annually, with another 240,000 living with the condition.
CannEpil has been developed for drug-resistant epilepsy which affects about 30% of all sufferers.
“I welcome news of this agreement. So much more is needed in terms of patients having access to authorised prescribers and a range of quality, legal, medicinal cannabis products, and this is a great step forward,” Epilepsy Action Australia chief executive officer Carol Ireland said.
“There is significant potential for cannabinoid therapies as a treatment option for people with epilepsy and many other conditions,” Ms Ireland added.
MGC Pharmaceuticals’ stock was up more than 5% in late afternoon trade, after spiking at $0.08c, or 14% higher, in early morning trade.
