Living Cell Technologies Parkinson’s disease treatment shows signs of efficacy in phase II study

Living Cell Technologies NTCELL Parkinson’s disease trial shows safety and efficacy at one year.
Treating nervous system-related conditions could soon be taking a technology-induced futuristic turn after biotechnology developer Living Cell Technologies (ASX: LCT) confirmed the safety profile of its NTCELL product, a potentially revolutionary method of treating debilitating conditions such as Parkinson’s disease and Alzheimer’s.
Following a phase 2b study of NTCELL, LCT says it has received official confirmation from the Data Monitoring Board, that there are no safety issues arising from the data.
In addition to Parkinson’s disease, NTCELL has the potential to be used in a number of other CNS indications, including Huntington’s, Alzheimer’s and motor neurone diseases including amyotrophic lateral sclerosis (ALS).
Over the past year, LCT has been working on a study on 18 patients who have been treated with NTCELL and says that there has been a “statistically significant improvement change” amongst the sample group in addition to no safety issues being recorded.
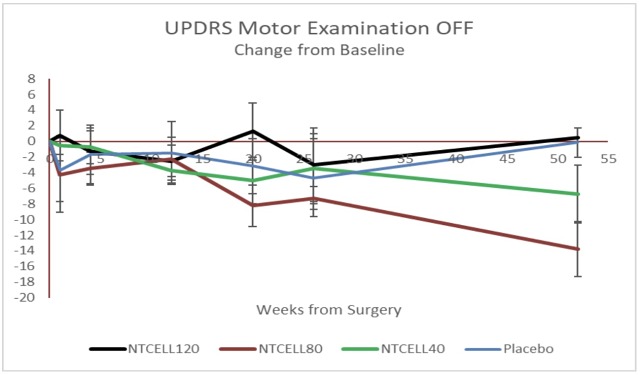
Efficacy data showing a statistically significant improvement in the Unified Parkinson’s Disease Rating Scale in patients who received 40 or 80 NTCELL.
“We are encouraged to see efficacy data at the longer time point. We need to further analyse this encouraging result at future time points to assess NTCELL as a potential treatment for Parkinson’s disease,” said Dr Ken Taylor, CEO of Living Cell Technologies.
Studying NTCELL
According to the biotech developer, the study was designed to confirm the most effective dose of NTCELL and to define any placebo component of the response.
The study’s patients had alginate coated capsules implanted on either side of the brain containing “neonatal porcine choroid plexus cells” which LCT says are naturally occurring support cells that have several benefits; including the secretion of cerebrospinal fluid (CSF), which support nerve cell functions and catalysing protective enzymes that are crucial for nerve growth and healthy functioning of the brain.
LCT says that after its NTCELL capsules are implanted into a damaged site within the brain, NTCELL functions as a “neurochemical factory” producing CSF and secreting multiple nerve growth factors that “promote new central nervous system growth” and “repair disease-induced nerve degeneration” while potentially removing waste products such as amyloids and proteins.
To date, LCT has put its NTCELL product through two studies and has filed a patent application in the US in December 2016.
Over the past 2 years, NTCELL has undergone a phase 1/2a clinical trial for the treatment of Parkinson’s disease in New Zealand and has met the primary endpoint of safety whilst “halting the progression of the disease three years after implant”. Results from that trial were used to design the larger phase 2b trial which is still ongoing.
The novelty behind NTCELL is that its component parts are sourced from “a unique herd of pathogen-free pigs” bred from stock originally discovered in the remote sub-Antarctic Auckland Islands.
LCT’s research has shown that NTCELL has the potential to treat neurodegenerative diseases because choroid plexus cells help produce CSF as well as a range of nerve growth factors that have been shown to protect against nerve cell death in animal models of disease.
“NTCELL has been shown in preclinical studies to regenerate damaged tissue and restore function in animal models of Parkinson’s disease, stroke, Huntington’s disease, hearing loss and other non-neurological conditions, such as wound healing,” LCT said.
“The treatment is safe. There are clinical signals of interest. We need to continue to monitor patients for longer to examine the clinical significance of this efficacy data,” said Dr Barry Snow, the study’s principal investigator from Auckland City Hospital in New Zealand.
