Incannex Healthcare (ASX: IHL) has taken another key step in its studies to help improve the comfort of millions of sleep apnoea sufferers and destruct a multi-billion dollar medical equipment industry.
The clinical-stage pharmaceutical company is preparing for the next stage of testing of a cannabinoid pharmaceutical product that it believes can reduce the use of expensive and uncomfortable medical equipment many users currently wear for safety and improved sleeping habits.
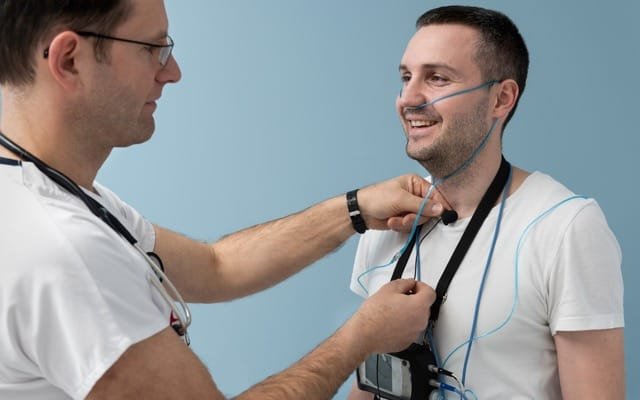
Obstructive sleep apnoea (OSA) occurs when a person has breathing pauses at night, because their throat becomes partly or completely blocked for a short time while they are sleeping.
In most cases of severe sleep apnoea, a doctor will recommend CPAP (Continuous Positive Airway Pressure) Therapy, or other oral devices that patients may find cumbersome and effect that sleep.
Multi-billion dollar market
Currently the global sleep apnoea devices market in terms of revenue is estimated to be worth US$5.8 billion (A$8.4 billion) in 2023 and is tipped to reach US$8.0 billion (A$11.6 billion) by 2028, growing at a CAGR of 6.5% from 2023 to 2028.
Melbourne-headquartered Incannex has developed a novel, pill-form, user-friendly treatment named IHL-42X that has already shown promise in early trials.
In preparation for the critical next phase, the company has brought in two highly regarded lead principal investigators (PIs) for an Investigational New Drug Application (IND) opening Phase 2/3 clinical trial to further investigate IHL-42X as a treatment for apnoea.
Wealth of experience leading the studies
Incannex chief executive officer and managing director, Joel Latham, says the two appointments Dr John Hudson of FutureSearch Trials of Neurology from Austin, Texas and Dr Russell Rosenberg of Neurotrials Research of Atlanta, Georgia, are two leaders in their field.
Dr Hudson has supervised over 300 clinical trials over the past 20 years mostly related to neurological and sleep disorders and has been a national and international speaker for these disorders.
Dr Rosenberg has more than 35 years’ experience in clinical sleep medicine and research, acting as an investigator in over 300 clinical trials including 14 in sleep apnoea and 211 in other sleep related disorders
He is a board-certified sleep specialist and Fellow of the American Academy of Sleep Medicine, Dr Rosenberg is former chair and spokesperson for the National Sleep Foundation (NSF) and has appeared frequently on local and national television news shows including the Today Show, Good Morning America, CNN, and MSNBC.
Another critical step
Mr Latham said recruiting the lead principal investigators is a critical step for the IND opening Phase 2/3 study.
Principal investigators focus on clinical research studies for treatment of neurological, pain and sleep disorders, which features the use of an on-site sleep lab.
“Our company is delighted to have the endorsement of two leading esteemed research scientists with the relevant experience to advance our IHL-42X drug candidate. Their support is testament to the efficacy of IHL-42X demonstrated in phase 2 proof of concept studies,” Mr Latham said.
“The multi-site clinical trial being arranged is a pivotal trial, meaning that it can be used for the registration of IHL-42X for regulatory registration and commercial launch, which is an exciting development for Incannex after much hard work and dedication from our broad team of contributors.”
Dr Hudson and Dr Rosenberg’s associated facilities will serve as the first clinical trial sites for submission to the US Food and Drug Administration (FDA) and for ethics approval from Institutional Review Boards.
Incannex plans to recruit approximately 45 sites across multiple locations for this critical trial in the US and other jurisdictions.
Current treatment options are limited
According to Dr Hudson, obstructive sleep apnoea affects millions of people and remains under treated.
“This is due in part to patients not being diagnosed, and in part due to poor patient compliance with current therapeutic modalities.”
“While unheard of a few years ago, oral medications to help reduce the cause of OSA, are now undergoing further investigation. This is more than exciting, it could prove to be life-changing for many patients.”
Dr Rosenberg says Incannex has developed a sound, rational, scientific protocol to determine the efficacy and safety of IHL-42X in subjects with obstructive sleep apnoea.
“Many sleep apnoea patients cannot adhere to positive airway pressure therapy, use it for an inadequate period at night or just refuse it. Having a safe, effective pharmacological option for obstructive sleep apnoea will be a positive addition to the treatment landscape as it will offer those that struggle to adhere to positive airway pressure therapy an alternative therapy.”
IHL-42X is identified as a proprietary synergistic composition drug formed from low-dose dronabinol (2.5mg), a synthetic form of Tetrahydrocannabinol (THC), and acetazolamide (125mg), a carbonic anhydrase inhibitor.
Phase 2 proof of concept clinical trials were previously undertaken by Incannex, with 44 data points from participants. Trial participants in the trial obtained a large and statistically significant reduction in the apnoea-hypopnea index (AHI) vs placebo.
Average apnoea hypopnea index (AHI) was reduced from 42.8 to 21.1 events/hour in the IHL-42X arm, a 50.7% reduction in AHI.
Additionally, 60% of participants experienced a greater than 55% reduction in AHI and 25% of treated participants displayed an 80% or greater reduction.