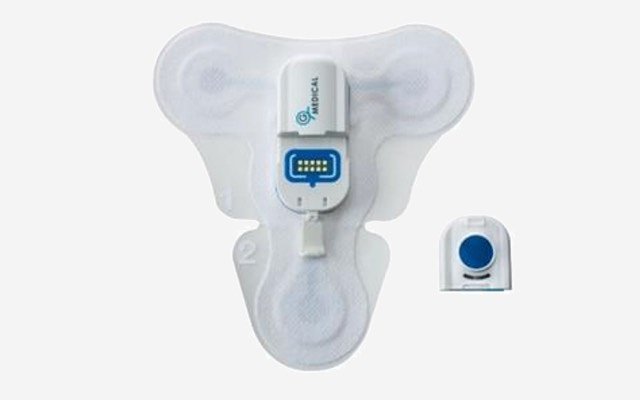
Medical device and telehealth company G Medical Innovations (ASX: GMV) has been granted approval by the Therapeutics Goods Administration (TGA) for its extended holter patch for the continuous cardiac monitoring of patients before and after hospital admission.
The company said the approval marks a significant milestone as the patch now officially complies with all relevant Australian medical and safety requirements as a Class IIa medical device.
TGA certification adds to the previously granted European CE Class II approval received in 2017.
G Medical is also pursuing required regulatory approvals in other target markets including the United States.
G Medical chief executive officer Dr Yacov Geva said the TGA approval demonstrates how the company’s suite of medical devices and telehealth technologies are relevant to the current environment.
“Our medical devices and telehealth platforms are becoming recognised as cost-effective and scalable solutions to ease the burden on healthcare systems, which can be adopted and utilised by the patient and their physician with relative ease,” he said.
“Our products are effective and relevant in the current COVID-19 environment and are important for monitoring and remote management of patients with chronic diseases as well as other specific medical indications, in parallel and beyond the pandemic,” he added.
Clinical-grade solution
The G Medical extended holter patch is part of the modular vital signs monitoring system (VSMS) and has been marketed as an easy-to-use clinical-grade device for patient management throughout the healthcare lifecycle.
It has been developed for use in clinics, assisted living residences, hospitals and with healthcare providers, and can streamline and simplify patient outcomes by delivering a continuous, 14-day recording of a six-channel electrocardiogram.
“The GMP seeks to streamline patient management across all stages of the healthcare lifecycle, including pre-hospitalisation, admission and post-discharge,” Dr Geva said.
“The product allows for a higher level of patient care, takes the burden off medical personnel, increases efficiency, decreases liability and [can] reduce the number of re-admissions,” he added.
At mid-morning, shares in G Medical were trading 17.02% higher at $0.11.