Creso Pharma (ASX: CPH) is on track to complete its Canada-based medical cannabis growing facility by mid-2018 in time for new legislation allowing edible cannabis products to be sold in the country.
The new 20,000 square foot facility is due for completion by mid-2018 and is expected to produce up to 4,000kg of cannabis annually. The facility will operate to GACP and GPP standards to enable Creso to expand its research and development.
Creso will own the facility through its 100%-owned subsidiary Mernova Medicinal Inc, with the duo exercising the option to purchase 9.75 acres in adjoining land to expand the facility up to 200,000 square feet in the future.
One the future 200,000-square foot facility has been developed, Creso anticipates annual cannabis production up to 40,000kg.
Creso has put in an application for a medical cannabis cultivation licence which, once granted, will make it one of the few companies in the world to cultivate, extract and manufacture its therapeutic cannabis products for animals and humans in Canada.
Under Canada’s amended legislation, edible cannabis products will be allowed to be sold in the country from 2019, with Creso planning to target that market with its cannaQIX and cannapeal ranges.
These nutraceutical products have global export authorisation and are believed to alleviate anxiety and stress.
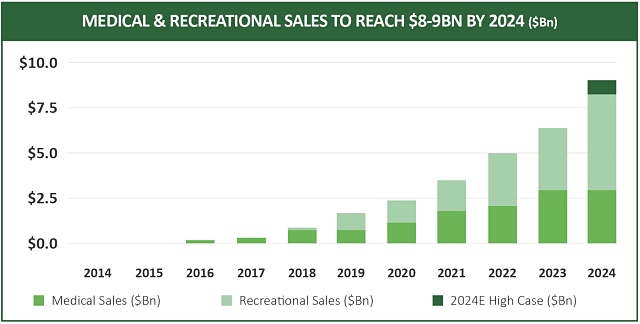
By June 2017, more than 200,000 patients in Canada were registered for medical cannabis, up from 75,000 the previous year.
Both Creso and Mernova have teamed up with 16 licenced cannabis producers to help develop Canada’s new cannabis advertising standards before recreational use becomes legal in 2018.
Earlier this month, equity analyst Morning Star released a report on Creso indicating the company’s stock was undervalued at A$0.57 per share, projecting the sector’s mean share price at A$0.92.
