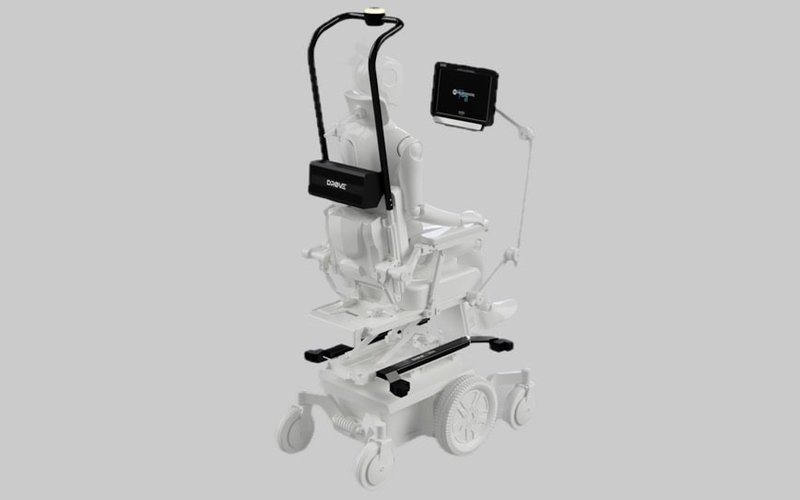
An autonomous wheelchair module developed by Control Bionics (ASX: CBL) will be launched on the local market after being listed on the Australian register of therapeutic goods.
The DROVE mobility solution is now classified as a Class 1 medical device following approval by the Therapeutic Goods Administration.
This status comes after 12 months of extensive internal and external testing by Control to ensure the highest standards of safety and effectiveness.
Improved independence
Manufactured in Australia, DROVE aims to provide powered wheelchair users with a new level of independence and ease of use within their own homes.
It has been developed for patients whose ability to communicate is compromised by conditions such as motor neurone disease and amyotrophic lateral sclerosis (ALS) and is designed to integrate seamlessly with existing wheelchair systems, enabling users to navigate their environment autonomously and safely.
Chief executive officer Jeremy Steele said that DROVE would herald a new era in assistive mobile technology when it is officially launched in the coming weeks.
“Our team has worked for years to design, test and get this device registered to benefit Australians who live with conditions that impact their ability to control their own wheelchair,” he said.
DROVE benefits
Many people living with ALS use powered wheelchairs at some point after their diagnosis, particularly in the mid-to-late stages of the disease.
Conventional powered wheelchairs, which offer navigation via a joystick (or alternative), can only move in relatively open spaces and do not allow patients to make the necessary movements to control or direct the wheelchair safely or in a timely fashion.
The DROVE self-driving module allows people with advanced ALS to move safely and independently around their home or other locations by using multiple assistive technology inputs to select from an array of pre-determined locations.
Once selected, the wheelchair will independently traverse a path—including turning, entering doorways and reversing—without the need for any additional user input before stopping at the target location.
The module has built-in sensors to detect obstacles, which then trigger the system to independently stop the wheelchair.
US market approval
Control’s American subsidiary is now working on achieving approval for DROVE from the US Food and Drug Administration for sales into the company’s largest potential market.
The work is being supported by a $570,000 research grant awarded to Control in January by the ALS Association that is expected to fund the examination of DROVE’s installation and usage procedures for technicians, families and caregivers.
Funds will also be allocated towards testing navigation and collision avoidance functionalities in multiple real-world settings and the creation of upgrade packages for different wheelchair types.
“The US is a key market for Control,” Mr Steele said at the time.
“It represents a large market for our autonomous wheelchair product and we are […] delighted that the ALS Association has awarded this grant in support of our efforts to bring this groundbreaking technology within reach of US patients.”