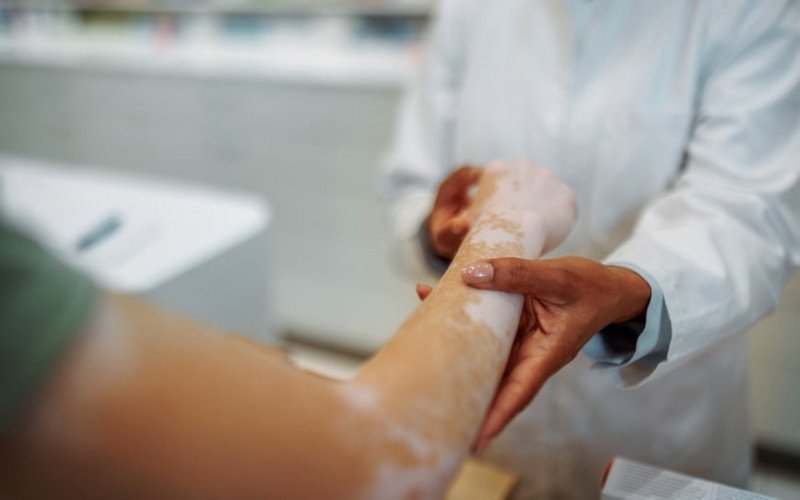
Clinuvel Pharmaceuticals (ASX: CUV) has recruited more than 200 patients for a Phase III trial (CUV105) of its breakthrough Scenesse (afamelanotide 16mg) repigmentation therapy to treat vitiligo.
The company is conducting the randomised 20-week trial at 37 sites across North America, Africa and Europe, with 57% of patients enrolled in the US.
The last patient to enter CUV105 will complete screening this month and Clinuvel expects first results in late 2026.
Enrolment milestone
Clinuvel director of global clinical affairs Dr Emilie Rodenburger said the company was “thrilled” with the enrolment milestone.
“We are essentially establishing a North American distribution network among dermatologists, anticipating the necessary infrastructure ahead of market entry for our breakthrough product,” she said.
“The first clinical observations of our systemic solution have been encouraging and we will continue regulatory discussions in Europe, Africa and North America in anticipation of CUV107, which will be our second large Scenesse trial.”
Repigmentation therapy
Scenesse is being evaluated as a systemic (or total body) repigmentation therapy for vitiligo patients, with a clinical focus on adolescents and adults with darker skin types.
Patients will receive treatment every three weeks—either with Scenesse alongside adjuvant narrowband ultraviolet B (NB-UVB) phototherapy administered twice weekly for five months, or NB-UVB monotherapy twice weekly for the same period.
Patients assigned to NB-UVB monotherapy will be eligible to receive Scenesse and adjuvant NB-UVB after completion of the follow-up period.
VASI evaluation
The study will use the Vitiligo Area Scoring Index (VASI) to evaluate the primary endpoint, which is a minimum of 50% repigmentation across the total body surface area.
Secondary endpoints include evaluations of repigmentation on the face, neck and head at week 20, and the maintenance of repigmentation following completion of treatment.
All patients will receive follow-up treatment for six months after the study.
Past case studies
Four previously released case studies of Scenesse on patients with skin type IV and varying disease duration demonstrated repigmentation of vitiliginous lesions on their face or back after four weeks of commencing treatment.
Some patients experienced additional spontaneous repigmentation following the conclusion of the treatment protocol.
A fifth case study has demonstrated repigmentation of lesions on the arms and legs in a skin type V patient with a 20-year history of vitiligo.
All patients reported satisfaction with the treatment results and tolerated afamelanotide with adjunct NB-UVB.
Acquired disorder
Vitiligo is an acquired depigmentation disorder affecting around 2% of the global population that causes progressive loss of functional melanin-producing skin cells.
While the disease impacts all sufferers, it affects those with darker skin types most severely, including an estimated 820,000 individuals across North America.
Only one approved topical immunosuppressant is currently available to patients in the US and Europe with vitiligo on less than 10% of their body surface area who have previously failed transdermal formulations.