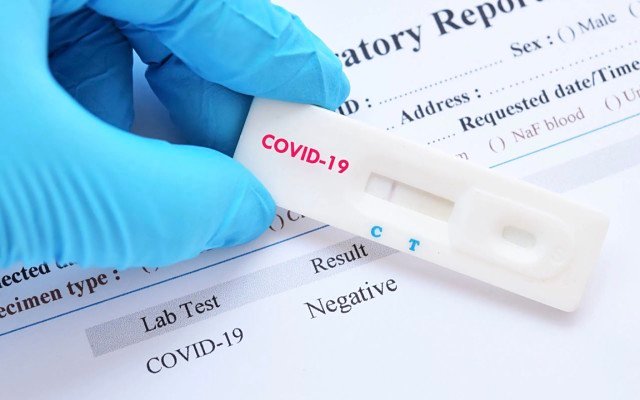
Life sciences company Cellmid (ASX: CDY) is set to come out of its self-imposed trading halt on Monday with news that it will commercialise a rapid test capable of detecting coronavirus (COVID-19) within 15 minutes.
First developed in China by biotech company Guangzhou Wondfo Biotech, the diagnostic test is set to be used as a bedside point of care tool, in doctors’ surgeries, pathology labs and in remote sites administered by healthcare professionals.
In a statement late on Friday, Cellmid said it had signed a supply agreement for the rapid diagnostic test with the authorised distributor, Australia Applications, with Wondfo Biotech now set to mass manufacture the test and supply it to Australia, for which Cellmid will pay a fixed price for each test.
Cellmid declared that it had placed its first order, while authorised distributor Australia Applications had issued its first invoice to kick off the deal for an initial period of 1 year.
The COVID-19 rapid diagnostic test was fast-tracked as a point of care test by the Chinese NMPA on 24 February, received the CE mark on 5 March and obtained approval from the Australian TGA on 25 March this year.
In total, the test obtained certification in all three jurisdictions in just over 1 month as government response measures including relaxed administrative measures were implemented in response to the COVID-19 pandemic.
According to Cellmid, the rapid diagnostic test is already used in several countries including the UK, Belgium, Spain and Germany.
Despite the test’s functionality, Cellmid said it has not entered into any agreement to sell the tests to customers yet but may enter into trade finance, vendor finance or prepayment arrangements with customers should it be required to fund larger purchases.
Rapid response
In a bid to counter the continual spread of COVID-19 globally, several medical technology companies set out to develop a test that could ultimately ease the strain on public health officials and provide a better understanding of past and present infection rates.
Wondfo’s COVID-19 rapid test is a small disposable kit that uses a “lateral flow colloidal gold-based detection” method against viral-specific IgG/IgM, delivering results “in no more than 15 minutes” and requiring only the most basic of laboratory equipment.
Currently, existing tests require several days or even weeks due to the need to send them to external laboratories.
On the contrary, the test Cellmid will market in Australia consists of a small device that requires only 10 microlitres of patient serum or plasma, or 20 microlitres of whole blood, to be loaded into a receptacle, alongside an included buffer, which then mixes with viral S protein fragments and migrates along the device to an area of immobilized capture antibodies.
If virus-specific IgG or IgM is present, conjugates are formed, which show up as a distinctive red band on the device within minutes.
Most other available COVID-19 detection methods make use of PCR technology to detect viral RNA which requires skilled technicians, takes several hours to produce a result and is limited in throughput by the availability of specific laboratory equipment.
In terms of efficacy and validation, Cellmid cited clinical validation studies first completed by Wondo that used 596 clinical samples and showing specificity of 99.57% and a sensitivity of 86.43% on day three and 95% on day five of infection.
“Cross comparison of gold standard PCR based testing with the device showed a 94.93% coincidence, proving that the device is positioned as an excellent rapid screening tool,” Cellmid said.
Technical validation studies have shown no cross-reactivity with major respiratory pathogens, no interference from common biological confounders and a kit to kit and intrasample precision of 100%.
“Learning from countries that managed the coronavirus infections well it is clear that widespread COVID-19 testing, isolation of those testing positive and early treatment are the best methods to control the spread of infection while saving lives and medical resources,” said Maria Halasz, chief executive officer of Cellmid.
Overarching business
Aside from its COVID-19-focused commercial activities, Cellmid published an operations update and said its consumer health product sales in Australia and Japan have been “moderately impacted over the past few weeks”.
The life sciences company confirmed that sales in Australia represent around 23% of its total which had “performed well until the end of February”, However, the company’s highly-anticipated product launch of Priceline planned for March has been delayed and “will start effecting sales from April”, according to Cellmid.
In Japan, Cellmid generates 68% of its total sales and said it expects Japanese operations to deliver on budget for the second half of the 2020 financial year, with slightly increased export and lower domestic sales expectations.