Biotron rides wave of Phase 2 clinical trial success for HIV drug

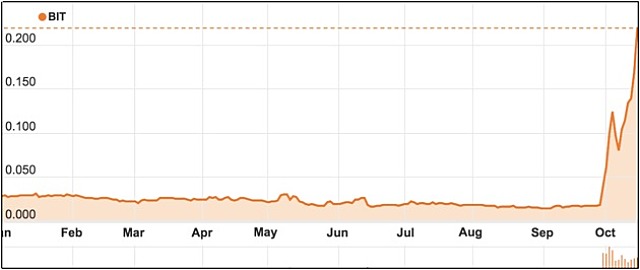
Shares in Biotron are up over 950% since the release of its Phase 2 HIV drug trial results less than a month ago.
Biotechnology company Biotron (ASX: BIT) is enjoying its moment in the sun on the back of a skyrocketing share price since news of a successful Phase 2 clinical trial for its BIT225 HIV drug hit the market last month.
The company’s stock has soared over 950% since the release of trial results on 28 September, which showed significant immunological benefits in patients receiving antiretroviral drugs with 200 milligrams BIT225, compared to antiretroviral drugs plus placebo.
The data was consistent with targeting and eradication of the HIV virus from macrophage reservoir cells where the virus is believed to live.
It showed that BIT225 attacks HIV-1 growing in macrophage cells, resulting in the production of a replication-incompetent (or non-infectious, dead) virus recognised by the body’s immune system and triggering a range of changes to the immune cells which fight disease.
The headline results indicated BIT225 had a “profound effect” on a source of virus which persists in the presence of antiretroviral drugs.
Eradication of the virus, produced by long-lived reservoir cells, is central to an eventual HIV cure strategy.
Next stage funding
Biotron will now require funding to proceed to the next stage of development.
The company is preparing to brief potential pharmaceutical partners on the BIT225 HIV-1 trial outcomes and hopes to progress commercialisation negotiations.
It will also continue exploring regional partnering opportunities for its ongoing BIT225 Hepatitis C (HCV) program in China, which has one of the world’s largest populations of people infected with HCV.
Pivotal stage
The cost of worldwide HIV patient treatment has been estimated at over $20 billion per year, representing a significant burden on healthcare systems.
The success of Biotron’s Phase 2 trial has been regarded as a pivotal stage in the fight against HIV.
Biotron said the important trial builds on a “solid foundation” of previously-reported laboratory studies which show that BIT225 attacks HIV-1 in macrophage cells which current drugs cannot access.
In order for HIV to be properly cured, macrophage cells must be cleared of the HIV-1 virus.
“Current drugs do an excellent job of taking HIV-1 in the blood to undetectable levels but do not clear [the] virus in macrophages,” the company said.
“These cells, which reside in the body’s tissues, produce low levels of HIV-1, even in patients taking current antiretroviral drugs [meaning] that the infection persists.”
Patients cannot cease antiretroviral drug treatment – if they do, the virus will quickly rebound to high levels.
Clearing out the virus from macrophage cells is an important key step towards the complete eradication of HIV1.
Tax incentive
Biotron has announced the receipt of a $1.07 million rebate for its research and development efforts from the federal government.
The rebate falls under an incentive program which allows companies to receive cash refunds for 43.5% of eligible expenditure on research activities and in this case, relates to Biotron’s antiviral drug developments.
“While we are fully-funded for current activities, this rebate will strengthen [our] cash position and support our commercialisation activities,” said managing director Dr Michelle Miller.