On the eve of its ASX debut, biotech company Atomo Diagnostics (ASX: AT1) has revealed it has received additional orders for its proprietary rapid blood test devices being used to test for COVID-19.
The company today announced a second purchase order from French diagnostics company NG Biotech to supply a further 550,000 devices to test antibodies produced in response to the COVID-19 virus, taking the total number of device orders to 947,200.
The news comes after Atomo was accepted into the ASX official list on Tuesday. The company is expected to commence trading on Thursday with an undiluted market capitalisation of $112 million (at the initial public offering price of $0.20 per share).
This will make Atomo the first company to list on the Australian exchange since 27 February, after successfully raising $30 million in its IPO.
Speaking with Small Caps, Atomo co-founder and managing director John Kelly said Atomo is the “right” company to be entering the market now.
“It’s a difficult market to be listing in, there’s no doubt about that, but I think our business is growing and we’ve got a very good solution for COVID-19,” he said.
Device delivery
Mr Kelly said NG Biotech’s first order, received four weeks ago, has already been dispatched and the latest order covers supply from May through to July.
The rapid diagnostic test (RDT) devices are CE Marked for professional use for COVID-19 testing throughout Europe.
NG Biotech has commercial rights to sell in France and the United Kingdom at the moment, according to Mr Kelly.

He said the company is moving “very quickly” to respond to the interest in its rapid test devices “for delivery of reliable, user friendly rapid blood-based testing as part of the global response to the COVID-19 pandemic”.
“Focus is currently on fulfilling the first two orders placed by NG Biotech, who are already supplying tests to the French Ministry of Defence,” Mr Kelly said.
Under a current supply deal, NG Biotech has the right to purchase up to 2.465 million of the devices during the 2020 calendar year.
The pair are expected to enter into a further binding purchase agreement for the ongoing supply of Atomo products beyond 2020.
“In parallel, we remain in discussions with two US-based COVID-19 test manufacturers for supply of [Original Equipment Manufacturer] test devices,” Mr Kelly added.
Same technology used for HIV testing
Atomo’s rapid test platform was initially developed as a HIV screening test and is the only HIV product approved in Australia for self-test use.
Since 2015, the company has sold more than 550,000 RDTs to medical professionals and consumers, plus a further 430,000 to other RDT manufacturers for sub-assembly.
“We’ve got the only HIV self-test and it’s the same rapid test platform that’s being used now for COVID-19, which is why we’re very confident about its usability,” Mr Kelly said.
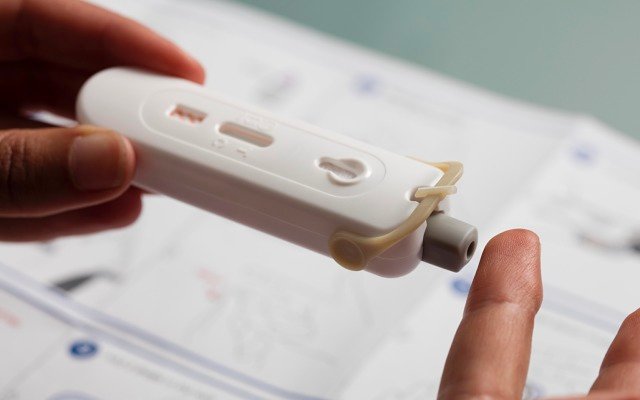
Atomo’s devices are designed to turn around results within 15 minutes by using a small drop of blood from a finger sample.
“There are other rapid antibody tests out there, but other companies have commercialised a ‘bits in a box’ chemistry set, so doing the test is kind of like a year 12 science experiment; whereas, Atomo has actually developed a consumerised single device that integrates all of those steps and automates a lot of it,” Mr Kelly said.
“It makes it a lot more reliable and simpler for a user to do a test quickly and easily,” he added.
In today’s announcement, Atomo said it will accelerate the planned expansion of its production capacity in order to meet the anticipated ongoing demand for COVID-19 test devices and continue to support the company’s existing HIV distributors and other manufacturing customers.