Althea continues medicinal cannabis industry expansion mission in Europe

Althea’s MyAccess Clinics’ Bristol site has received a licence from the Care Quality Commission.
Althea Group (ASX: AGH) continues to make inroads into the European market with operations in both the UK and Germany now online, supported by its proprietary technology platform Althea Concierge.
The medicinal cannabis company is also making commercial progress closer to home in Australia with over 3,000 patients now on its books, almost 500 of which were acquired in October this year.
Althea said that a total of 2,815 patients had been prescribed Althea’s medicinal cannabis products in October, representing a huge 20-fold year-over-year increase on the 127 patients recorded at the end of October 2018.
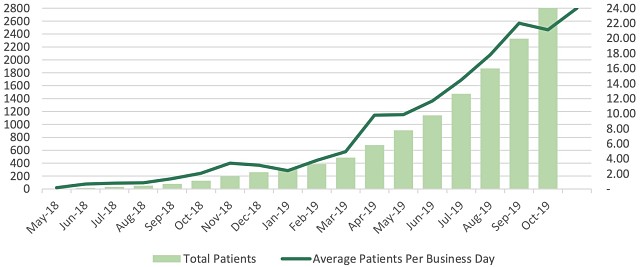
Althea has reached 3,000 patients in Australia, with 486 new patients added in October.
Moreover, Althea declared that around 375 healthcare professionals in Australia have now prescribed its products which have propelled the company to make significant strides towards building a medicinal cannabis supply line to a growing number of patients in Australia.
As a result of its sustained growth, Althea crossed the 3,000-patient milestone in November and said it was “on track” to reach 4,000 patients by the end of this year.
“It is hard to believe that just over a year ago Althea medicinal cannabis products had only been prescribed to 127 patients. We have seen phenomenal growth since then and are very pleased to have now passed the 3,000-patient milestone,” said Althea’s chief executive officer Josh Fegan.
European inroads
The momentum being generated in Australia is also being emulated overseas in vibrant new markets such as the UK and Germany.
Earlier this week, Althea signed a memorandum of understanding with Germany company Nimbus Health which will see its products sold and distributed across Germany via Nimbus’ pharmacy network.
According to Althea, Nimbus holds a wholesale pharmaceutical licence and estimates that it has direct access to approximately a quarter of all medicinal cannabis patients in Germany through its network.
Under the agreement, Nimbus will sell and distribute Althea’s full suite of pharmaceutical-grade medicinal cannabis products with both companies agreeing to work jointly to establish yearly sales targets, marketing and commercial strategies and developing “real world anonymised patient data”, Althea said.
Meanwhile, in the UK, Althea announced that it had reached an important milestone after its subsidiary, MMJ Clinic Group MyAccess Clinics, was registered and licensed by the Care Quality Commission (CQC), a British independent regulator of health and social care in England.
The MyAccess Clinics site in Bristol will now become only the second medical cannabis clinic in the UK, and the first located outside of London, to be granted a CQC licence. CQC registration allows for domiciliary care, meaning MyAccess Clinics prescriptions are now available for home care services.
“We are replicating our Australian strategy in the UK and German markets and expect to see similar patient growth trajectories in due course,” said Mr Fegan.
“Althea is in a market-leading position due to our brand recognition and high-value proposition which resonates with doctors and patients alike. The company remains fully funded and on track to deliver Australian manufactured Althea products in 2020, as well as supporting our international expansion,” he added.
Clinical trial supply
Another string in Althea’s bow is to supply medicinal grade cannabis to clinical trials – yet another avenue of expansion for the company.
On Wednesday this week, Althea said that that it had signed an agreement with the Australian Centre for Cannabinoid Clinical and Research Excellence to supply product for the Cannabinoid for Symptom Control in Advanced Cancer, an open-label prospective clinical trial in New South Wales being conducted by the University of Newcastle.
According to the terms of the agreement, Althea will serve as one of several suppliers providing two cannabis medicines on a commercial basis for the upcoming trial that will involve as many as 600 patients.
“We are big believers in the potential benefits of medicinal cannabis and pleased to support an initiative such as this, which will further our scientific understanding of its use in areas of unmet needs,” said Mr Fegan.
