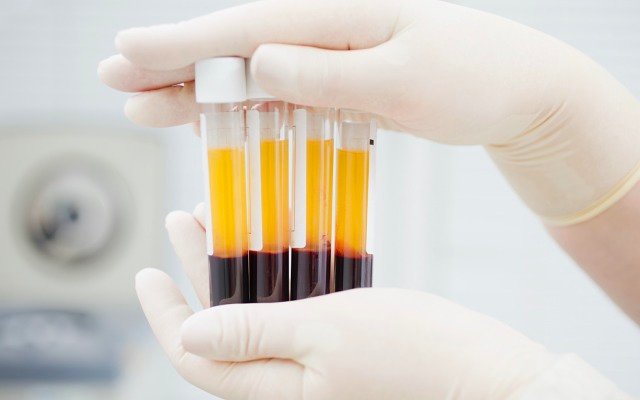
Australian bio-pharma company Aegros is looking to defend against COVID-19 and other diseases using its disruptive plasma processing technology to develop hyperimmune therapies that can protect front-line workers, the unvaccinated, and hospitalised patients, among others.
Aegros founding managing director John Manusu said plasma fractionation has essentially been unchanged since it was invented in the early 1940s.
The process involves separating components of blood plasma, to produce antibodies and other products that can then be administered therapeutically.
“These are lifesaving products that are used in haematology, immunology and neurology,” Mr Manusu explained.
“They are essential medicines this country needs.”
Plasma fractionation
Aegros co-founder and executive chairman Dr Hari Nair pointed out the conventional plasma fractionation method is multi-step and uses temperature, alcohol and “many other factors” in precipitating proteins that can then be fractionated into plasma.
“The most important factor here is it includes many steps, and, ultimately, a longer time with heavy losses in final production of the protein,” he said.
Patented and disruptive process
Dr Nair is a trained haematologist who co-invented Aegros’ patented HaemaFrac process, which he says will disrupt the way plasma fractionation is carried out.
In contrast to the multi-step conventional method, Aegros’ HaemaFrac technology is only four steps.
“It has one capture step, and it does it very rapidly.”
The system comprises three membranes: one at the top, the middle, and the bottom.
“The protein molecules are charged negative or positive. When the electrical field is turned on you get the transfer of the molecule or the positive haemoglobins into the lower stream for collection.”
Although HaemaFrac is only a four-step process, Dr Nair says it generates a higher yield, increased purity and comes with lower running costs.
Small volumes of plasma can be processed in hours via HaemaFrac, compared to thousands of litres which usually takes a week using conventional methods.
Additionally, HaemaFrac does not require temperature or alcohol, which reduces its environmental impact.
As a result, HaemaFrac gives Aegros the ability to produce far more doses at a faster rate of “lifesaving hyperimmune products” than other plasma fractionation companies including Australia’s only other producer CSL (ASX: CSL).
COVID-19 and Covimmune
The COVID-19 pandemic has given Aegros the opportunity to showcase its HaemaFrac technology in developing its first critical hyperimmune product called Covimmune.
When someone contracts COVID-19, their immune system enters a state of hyperactivity to fight the disease.
Those that recover have developed antibodies to the virus.
With its HaemaFrac technology, Aegros has been able to harvest these antibodies to produce Covimmune, which can be administered to frontline workers and others in need.
Once received, these people gain passive immunity and immediate protection from COVID-19.
An advantage to passive immunity is that it is immediate and lasts for a few weeks (or months at best).
Meanwhile, active immunity such as that from a vaccine can take up to several weeks to develop, although it does give longer-term protection.
Commercialisation pathway
Aegros has already installed the HaemaFrac system within its 4,500 square metre plant in Sydney with initial preproduction runs completed in January this year.
Australia’s Therapeutic Goods Administration finished an audit of the process in May with the good manufacturing practice (GMP) licence expected in the “near term”.
The GMP licence will pave the way for Aegros to begin manufacturing and penetrate the global therapeutic plasma market with Covimmune.
Meanwhile, Aegros has secured ethics approval to carry out its Covimmune clinical trial at Sydney’s Royal North Shore Hospital.
This phase 1-2 trial aims to establish safety and efficacy of Covimmune by comparing it to Covid-19 convalescent plasma.
The trial will involve a study of 30 healthy people over a 42-day period.
Australian Red Cross LifeBlood has provided the plasma for the convalescent arm of the study, which is now complete.
Once GMP approval has been secured, the Covimmune arm of the study will kick-off, with results anticipated before the end of the year.
“It is expected Covimmune will induce passive immunity in trial participants,” Aegros said.
Following a successful study, Aegros will apply to have Covimmune on the Australian Register of Therapeutic Goods – enabling it to be sold in Australia.
“With an expedited application process, Aegros anticipates registration will be completed within three months of the clinical trial,” the company noted.
Launchpad into billion-dollar markets
Aegros’ strategy is to use Covimmune as its launch pad to penetrate Australia’s $470 million therapeutic plasma market and the global market which is estimated at $19 billion.
The company is completing commercial due diligence with a Middle Eastern government regarding construction of a 100,000L HaemaFrac facility in that country.
A government submission is expected to be lodged by the end of the year, with the design and construct phase anticipated to begin early next year.
As well as producing Covimmune, the company plans to use its technology to create hyperimmune products for other diseases and illnesses ranging from rabies to the middle eastern respiratory virus, also known as MERS.
In conjunction with University of New South Wales’ Kirby Institute and the University of Queensland’s Institute of Bioengineering and Nanotechnology, Aegros is applying for a $65 million grant to build a greenfield HaemaFrac facility that can process up to 1 million litres of plasma a year.
Aegros is also discussing a possible collaboration with the NSW and Queensland governments. Additionally, it is working with IndigiMed to look at supplying cold chain solutions to remote indigenous and farming communities throughout Australia.
Capital raising
Aegros recently completed a private placement for $2 million and is seeking another $5 million at $2.30 per share with each share having one attaching $2.30 option that expires in March 2023.
The company has also undertaken a buy-back of convertible notes that were issued 12 months ago and is now giving investors the opportunity to participate in a $5 million shortfall.
Sydney based broker Pulse Markets is conducting the capital raising.
A wider opportunity to secure a stake in Aegros will be provided next year with the company planning an IPO and ASX listing.